 |
 |
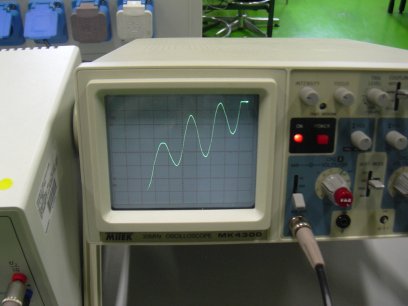 |
Carriers of negative "electricity", i.e. electrons are named after ancient Greek amber. So-called cathode-rays were identified as beams of free electrons by JJ Thompson in 1897.
Electrons are everywhere, carrying the electrical current in a wire, fluxing in gas neon lamp, attaching to electrodes inside a cell-phone battery, drawing a picture on the TV screen.
 |
 |
 |
Scientists used electrons to find that particles are waves, to study the structure of crystals, to find quarks inside proton.
Many other constituents of matter, like neutrons, protons and "exotic" particles were discovered since 1897, but electrons seem to be the only really small (1), elementary (2) and stable (3).
(1) An easy, classical way to determine the radius of electron is to compare its mass (m=9.1x10-31 kg) with its electrical charge (e=1.6x10-19 C). If the whole charge were brought to the surface of a small sphere, than the electrostatic energy E = ke2/2r compared to Einstein's energy E=mc2 gives the radius of r=2.8x10-15m, 18,779 less than Bohr's radius of hydrogen atom.
(2) Particles of light, photons, are also elementary, but massless.
(3) Proton is also stable, but is made of 3 smaller quarts, quarks.
For electron and hydrogen radii see:
http://newton.ex.ac.uk/research/qsystems/collabs/constants.html