

Can an iron bullet go through a stainless steel football sphere? It's simply impossible! But not for electrons.
Electrons, much smaller (about 10-15 m in radius) than atoms (10-10 m) would be a nice method to probe atomic dimensions, giving an answer to the question of Albert Einstein, from his PhD thesis (and from his article on Brown's motion) - "what are real atomic dimensions?" (1).


A. Einstein, Annalen der Physik 17 (1905) 133-148
Phillip Lenard, in his experiment in 1895, before the date of the "official" discovery of electron, noticed that electrons (or better "cathode rays") are attenuated in gases, proportionally to their density. This is clear, as a more dense gas contains (probably) bigger molecules.

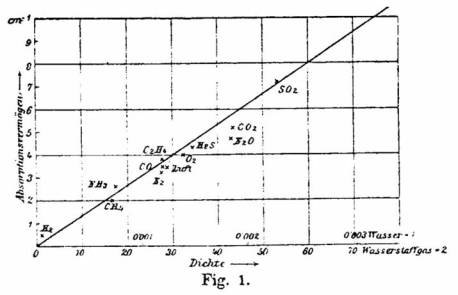
P. Lenard, Ann.d.Phys.u.Chem. N.F. 56 (1895)
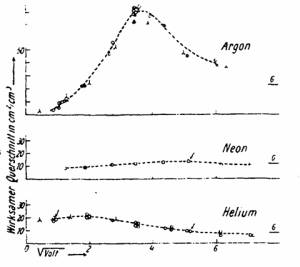
So far, so good. But the experiment of Phillip Lenard was done with fast electrons, at the energy of 3 keV. His countryman, Carl Ramsauer, in 1921 in Danzig (Gdañsk), now at the North of Poland, then Free Town, decided to study slow electrons. Much to his surprise (and of John S. Townsend, who did the same in England by another, so-called swarm method), in Argon, at a certain energy, the electron beam was not attenuated. Argon atoms were transparent!
[1] Ramsauer, C., Über den Wirkungsquerschnitt der Gasmoleküle gegenüber langsamen Elektronen, Annalen der Physik, 4, 64 (1921), pp. 513-540.
[2] Bailey, V. A., and Townsend, J. S., The abnormally long free paths of electrons in argon, Philosophical Magazine, S.6, 43 (1922), pp. 1127-1128.
 |
 |
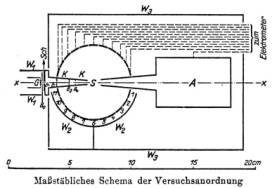
In Ramsauer apparatus the beam of electrons was forced by a perpendicular magnetic field to travel on a circle.
This turned to be a problem: why for electrons with 0.3 eV kinetic energy argon atoms (and krypton at 0.6 eV and xenon atoms at 0.7 eV) seem to be transparent?
Glass
is transparent, for the visible light (and non-transparent for infrared light).
Silicon is grey in visible light, but transparent in IR. But light is a wave,
and electrons are corpuscles, as earlier found by JJ Townsend.
Why electrons behave like waves? Are they waves?
This idea was so absurd, that a historian was needed, to risk an answer. His name was Luis de Broglie.
Yes, electrons are waves, and when they travel around a nucleus in the hydrogen atom, they form a standing wave.

Cafe Richard, rue Gay Lussac, Paris
L. de Broglie, Recherches sur la théorie des quanta, Thesis, Paris, 1924.
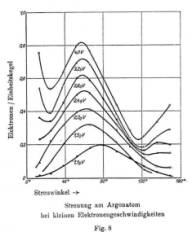
Carl Ramsauer came into scene again, in 1932, in Berlin. If electrons are waves, then should show "wavy" patterns, when scattered.

Lago Tovel, Trentino
In Berlin, C. Ramsauer measured angular distributions of scattered electrons on argon: they showed a wavy pattern!
 |
 |
But now it was not a surprise any more: E. Schrodinger already formulated his Quantum Wave Mechanics.
In quantum wave mechanics, a traveling electron is wave-like.

When it scatters on an obstacle, makes waves in all directions.
We describe the intensity of the scattered wave in a given direction ? by a following formula
![]()
where i is the imaginary unit, k is the "wave number" related to electron's energy, l defines electron's angular momentum, P are polynomials and h is so-called phase shift, i.e. the amount by which the incident wave retards (or anticipates) the non-scattered electron wave.
Now, you can see how experimental angular distribution can be approximated by changing the phase shifts of single partial waves which compose the wave of the scattered electrons (you do it by the potentiometers, the complex part exp(2i?) is shown on the ring and the angular distribution shown in the frames.)
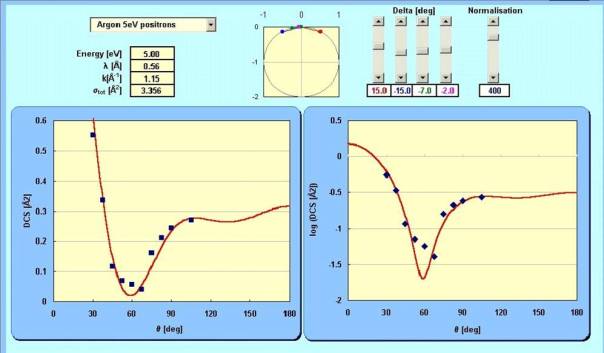
Programme by Helena Nowakowska (you can download it here)
Try to fix three potentiometers to zero, and change just one of them. See the shapes changing, depending on which potentiometer is non-zero. Like in real scattering.
See for example:
M. Allan, Measurements of the elastic and v=0›1 differential electron-N2 cross section over a wide angular range, J.Phys. B 38 (2005) 3655