Photoacoustics
Amounts of ethylene are insignificantly small - only 1 part over a billion of air, what requires special experimental methods. One is called photo-acoustics.
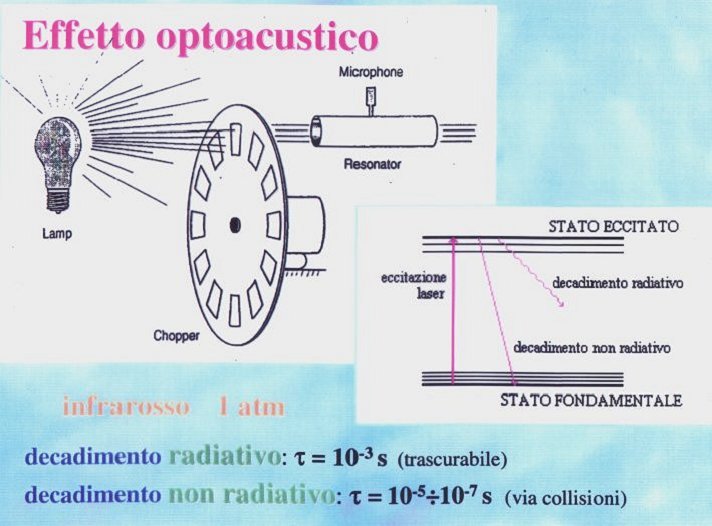
A beam of the infrared laser (CO2) excites vibrations of the ethylene molecule. Ethylene molecules collide with air molecules and transmit their excitation. If the laser is "chopped" with a frequency of a few kHz, a wave of faster moving molecules (a sound of this frequency) propagates in the gas – it is enough to use a microphone and we can hear
it.
A clue of the photoacoustic spectroscopy stays in the fact, that low-energy (i.e. long wavelength, i.e. infrared excitations) have longer lifetimes. For this reason, the decay from upper levels undergoes through collisions with other molecules, rather than through a direct radiative de-excitation. A sound is created.
 |
 |

Special thanks for schemes and explanations to Dr Andrea Boscetti CNR and IRST, Trento
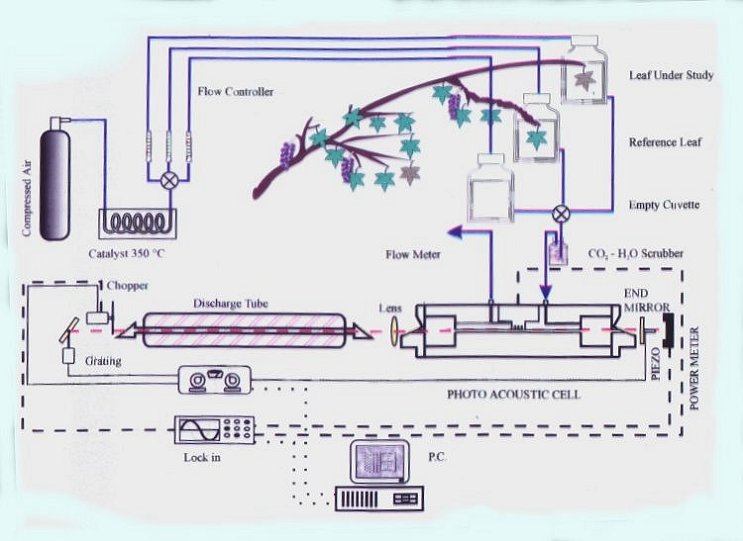
The sound is generated in the gas sample, if the radiative energy is absorbed, i.e. when at least one energy level in the searched molecule corresponds to the photon energy of the exciting laser. If more exciting laser lines are used and more levels correspond, we get a clear fingerprint of the molecules. Moreover, this method is particularly suited for complex molecules, with many vibrational levels.
The CO2 laser produce many strong lines in the infrared range - this type of laser is the most frequently in the photoacoustic spectroscopy. Nowadays, also semiconductor laser are used.
Ethylene molecule has many absorption lines corresponding to emission lines of the
CO2 laser.
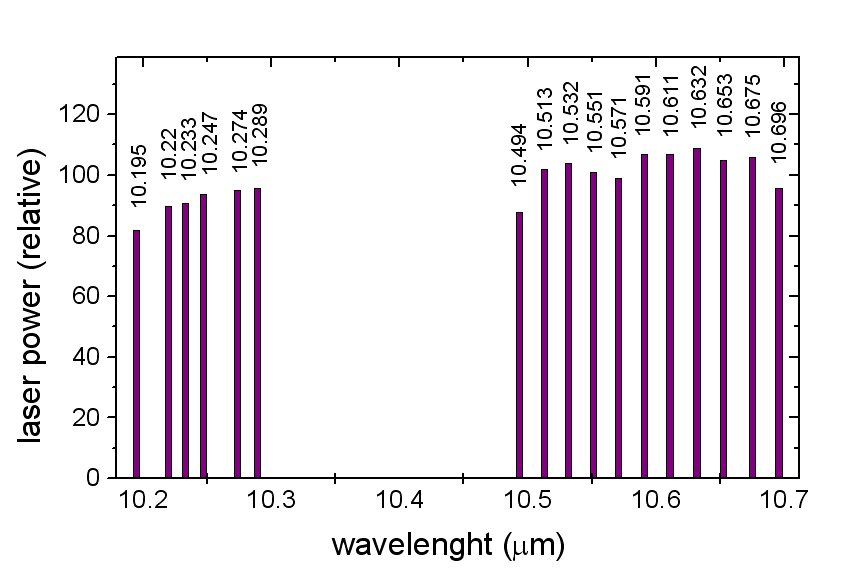
Spectral distributon of CO2 laser
 |
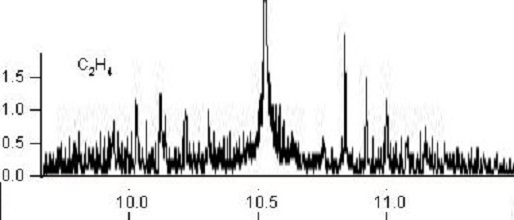 |
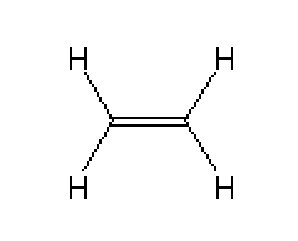
Ethylene molecule and its infrared absorption spectrum around the 10 μm wavelength
http://webbook.nist.gov/chemistry
http://vpl.ipac.caltech.edu/spectra/c2h4.htm
So, when photoacoustics will be available at home, after coming from work we will ask: |