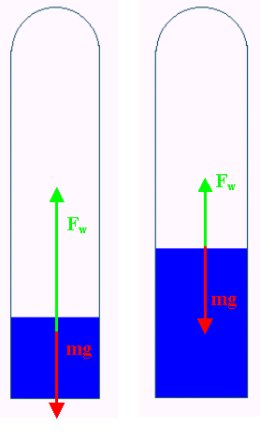
There is an eye dropper in a plastic bottle of water. The eye dropper is weighed in such a way that it is suspended with the opening directed to the bottom. There is some air trapped in the dropper.
If you squeeze the bottle, the pressure inside increases and, consequently the air pressure in the eye dropper also rises. If you examine closely, you will observe that the air bubble in the eye dropper gets smaller when you squeeze the bottle. This means that the diver's buoyancy has decreased as more water got inside. Unfortunately, the diver has drowned.
Modern bathyscaphs are equipped with floats filled with light petrol and not with air. If they were filled with easily compressible air they would behave like the Cartesian diver and hence would be useless. In order to construct your own Cartesian diver you need an old cork, a few small nails and an orangeade bottle. Good luck!
A Cartesian Diver dives due to the well known fact that, by contrast with air, water is almost incompressible. When we squeeze the bottle, the pressure inside it increases in value by Δp, Since the pressure increases, the volume of the gas bubble inside the diver decreases by ΔV. Thus, according to Boyle-Mariotte's Law
pV = (p +Δp)(V+ΔV)
Now it is quite easy to calculate the change in the volume of the gas bubble:
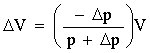
The diver's Archimedes force will decrease in proportion to the decrease of the volume of the gas bubble, or more precisely by ρgΔV, where ρ is the density of water and g is the gravitational acceleration. The diver begins to drown when his buoyancy becomes smaller than the force of gravity.