
Love Thermometer

It is easy to assess the changes in pressure between the upper and lower container of a Love thermometer. Let us assume that pg denotes the pressure in the upper container and pd the pressure in the lower container. After the liquid in the tube inside rises, the pressure volume on the lower surface of the liquid must be equal to the pressure in the upper container and the pressure of the column of liquid in the tube.
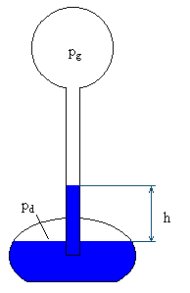
With alcohol density of ca. 800 kg/m3 , the difference in pressure of 80 Pa rises the liquid by 1 cm.
The rate at which evaporation process takes place depends on the difference between the pressure of saturated vapour pn at T T temperature and the actual pressure of vapour p, or more precisely:
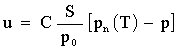
S denotes the surface of liquid, C is the coefficient characterising the specific liquid and p0 stands for external pressure, i.e. it is the sum of component pressure of individual gases which are not vapour. The relation between saturated vapour and the temperature is non-linear. It rises relatively quickly with the rise in temperature. For volatile liquids, whose boiling point is only slightly above the room temperature, even slight changes in temperature bring about considerable changes in pressure.