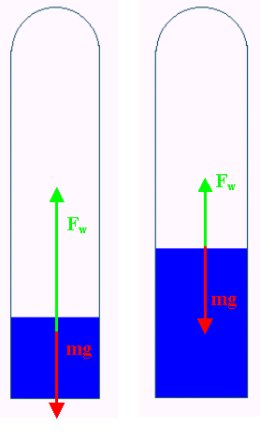
Cartesian diver

A Cartesian Diver dives due to the well known fact that, by contrast with air, water is almost incompressible. When we squeeze the bottle, the pressure inside it increases in value by Dp, Since the pressure increases, the volume of the gas bubble inside the diver decreases by DV. Thus, according to Boyle-Mariotte's Law
Now it is quite easy to calculate the change in the volume of the gas bubble:
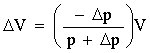
The diver's Archimedes force will decrease in proportion to the decrease of the volume of the gas bubble, or more precisely by rgDV, where r is the density of water and g is the gravitational acceleration. The diver begins to drown when his buoyancy becomes smaller than the force of gravity.