Soap Bubbles
How come (after adding detergent to water which considerably lowers its surface tension) the dishes covered with a film of fat (which is insoluble in water) were washed with small amount of washing up liquid?
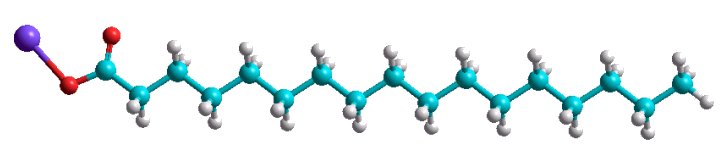
The detergent molecules consist of two different chemical parts joined by strong bond. One of the parts is usually hydrophilic (it attracts water) and the other is hydrophobic (most often it is carbohydrogen chain) for example sodium stearate CH3(CH2)16COONa (see the picture).

Such molecules are called amphiphilic (from Greek - "loving both"). After adding amphiphiles to, for example water with oil, hydrophilic molecules will organise molecules of water around themselves, whereas hydrophobic molecules will organise the molecules of oil, so as to prevent energetically disadvantageous contacts of water with oil from occurring.
In a similar manner, although glycerine mixes well with water (both compounds are strongly polar) adding detergents to such a mixture causes mutual reorientation of the molecules of water and glycerine, leading to more energetically favourable arrangement, and lowering the surface tension. Big soap bubbles can be produced.