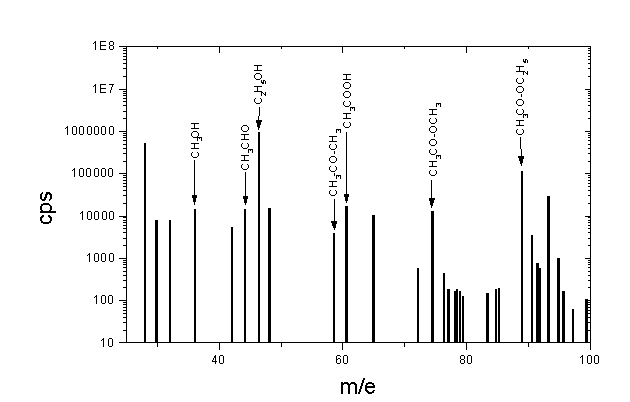
One of the problems in mass spectroscopy is the way in which molecules are ionized. Ionization can be done by eletrical discharge, laser light, electrons or another charged particles, but it usually leads to fragmentation of the molecules. In that case, finding out the original chemical composition of the sample from its fragments mass spectra is very difficult.
However, some ionization methods are "soft". One of them is a chemical ionization - by proton transfer. In that case the charge is transferred from previously ionized particles of the buffer gas, like methane, ammonia or isobuthan, to the studied molecules.
From recently, first in Innsbruck, hydronium H3O+ is being used for proton transfer ionizaion. These hydronium ions are created, for example during dissotiation of the protonated water clusters H+(H2O)n. Ionization process rely on transfer of the proton from hydronium to the neutral molecule M according to equation:
H3O+ + M = MH+ + H2O
As a result, the peaks in mass spectra appear shifted right by 1 atomic mass unit.
