Mass spectrometry consists in separating different atoms
(molecules, radicals) by their masses. More precisely, the ionized atoms or molecules of the substance
are selected accordingly to m/q ratio (m-mass, q-charge
of ion) and separately recorded by the mass spectrometer. The obtained mass
spectra allows to determine both the exact mass of the detected particles
and relative percentage quantity of this particle.
At the early stage of mass spectroscopy, the main task
was searching isotopic content of the elements and precise determination
of the atomic masses. From the first Thomson's experiments in the beginning
of the XX century, ths mass spectrometry has changed much. Now it is a useful
analytic tool, used in experimental physics, chemistry, molecular biology,
enviroment studies and so on..
Generaly, two main types of the mass spectrometers exist. In the first
one, the dependence of the trajectory of ions on the intensity of constant
electric (or magnetic) fields is registered (static mass spectrometers).
In second ones, the dependence of the time of flight of ions from their
source to collector or dependence of the ions vibrations in alternatic fields
is monitored (dynamic mass spectrometers).

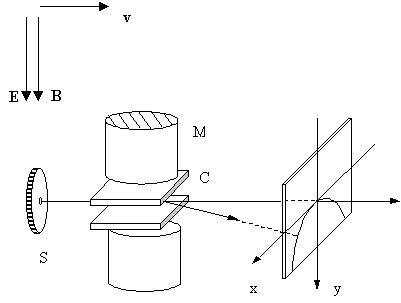
Beam of the ions, created in gas by an electric discharge, passes through electric field between two plates of capacitor and magnetic field oriented paralell to the electric field. In the plane of observation ions with the same charge and mass, but differing with velocities are placed along the parabola. Its vertex is in the point where ions not deflected by the fields would arrive.
The change of the y coordinate, caused by the electric field is determined by the following force equation:
![]()
Solution of this equation is:
![]()
A uniform magnetic field with induction B, directed along y axis, causes deflection of the beam into the x axis direction. Particles in this field are forced to move on circular orbits in plane perpendicular to the direction of the magnetic field. Because area of the magnetic field is limited, charged particles perform only a part of the circular orbit and then are moving on straight linea. From the condition of the balance between Lorentz's force and centrifugal force, the deflection of the particle into the x axis can be obtained:
![]()
From the equations describing x and y we obtain equation of the trajectory:
![]()
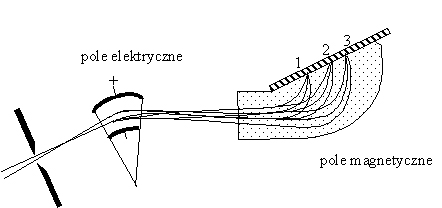
An improvement of the Thomson's mass spectrometer was presented in 1919 by Aston. He replaced paralell electric and magnetic fields by perpendicular ones. The beam is splitted by electric field according to m/e ratio, and also according to their velocities. Taking proper intensities of the fields we can cause that magnetic field directs all particles with differents velocities to the same point, leaving simultaneously splitted beams of the particles with different e/m ratio. Due to this fact spectrometer has a higher transmission of the ions and due to that - a higher resolution.
Time of flight analyzers (TOF) using dependence of time of thight of the ions from their e/m ratio. Let's suppose that ions after exiting the source are accelerated in the electric field by difference of the potentials Us and after passing of the distance d they reach the detector. Time of flight for an ion with m mass and q=ze total charge will be equal to:
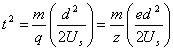 .
.
Mass resolution of the spectrometer is defined as value of the biggest detected mass, where is still possible to distinguish between ions differing with mass unit:
![]()
Resolution of the TOF spectrometers depends on many factors:
1) incorectness of the focusing rising from the fact that area where ions
are formed is finished (non zero),
2) broadening of the ion packet due to distribution of the angles of trajectories
of ions,
3) deflection from perpendicularity of the ions source in relation to
spectrometer axis,
4) effective depth of the channel detector plate,
5) effect of non equal initial velosity of the ions from the source,
6) non idal rectangular signal shape of th trihhering ions in the source,
7) broadening of the impulse in preamplifier,
8) influence of the remainder electric fields in area of the grids,
9) influence of the space charge created by ions charges,
10) time of duration of ionizing impulse.
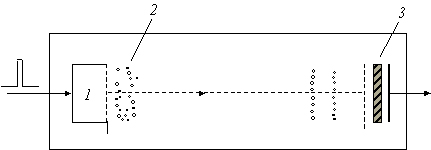
Schematics of the linear (single segment) TOF spectrometer. 1 -
ion source, 2 - ions packet in vicinity of the source, 3 - channel detector
of the secondary electrons
One of the major problems in correct sepermination of masses in time-of-flight spectrometers is correct determination of the start signal. Some set-ups uses flat transmission detector as a start detector: ion passing across this detector looses small amount of its energy. It gives start signal for time-of-flight measurement. The second, similar detector gives a stop signal. Disadvantage of that method is fact, that for heavy particles or light ones but with high energies on the first detector occurs multiple scattering. It causes at overall a decrease of the number of particles reaching the stop detector. Another method used to obtain the signal of start is photoionization, using short impulses of light.
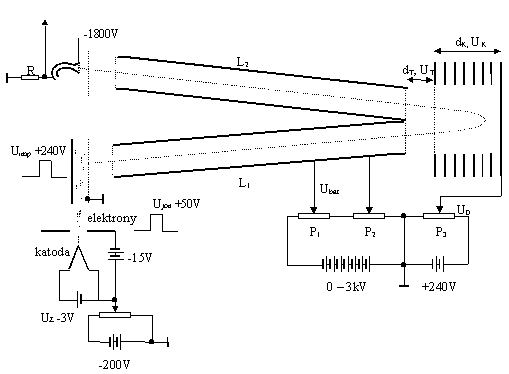
Schematics of the TOF spectrometer (TOF-Reflektron) from Institute
of Physics, Pomeranian Pedagogical Acedemy in Słupsk.
Improved version of the TOF mass spectrometer is TOF-Redlektron spectrometer build at Gdansk Polytechnic and used in Pomeranian Pedagogical Academy in Slupsk. An important improvement is the use of the array of electrodes arresting and reflecting ions into the detector placed close to the ion source. Due to this all advantages of the TOF spectrometers are kept, moreover, due to a much longer drift path, the spectrometer resolution is much improved. Besides, thanks to reflecting electric field, the ions differing in the initial energies while exiting the source but having the same m/q ratio, will reach the detector at the same time. It occures due to the fact that quicker ions enter deeper into the arresting electric field region and in spite of a fact, that after reflection ions still have higher kinetic energy in comparison to slower ones, they simoultaneously have a longer way to the detector. So, with proper adjustment of the electric fields time of flight of the ions with the same m/q ratio will be the same - independently on their initial velocity.

TOF spectrometer (Institute of Physics, PAP in Słupsk)
In quadrupole mass spectrometer, first time buld by Paul and collaborators in 1953, mass separation is obtained by using of electric field. Quadrupole mass analyzer is build with four long hyperbolic or circle electrodes (more you can read here)

Quadrupole mass spectrometer (Institute of Physics,
PAP in Słupsk)