
Seven top poisonous snakes live in Australia. They are terrifying in ways of killing and magnificent in their adapting to tough climates. As far as marine snakes kill in a rather similar way [1] the land snakes developed a whole range of mechanisms in a single venom shot. The most efficient and rapid way is just paralyzing the prey.
http://members.iinet.net.au/~bush/myth.html
Australia's Venomous Snakes: The Modern Myth or Are You A Man Or A Mouse?
by Brian Bush
LETHALITY IN MICE
From Broad, Sutherland & Coulter (1979)
(In descending order including LD50 in mg/kg administered subcutaneously. In first figure venom diluted in saline solution only, 2nd includes the addition of a blood protein, bovine serum albumin)
Inland Taipan (Oxyuranus microlepidotus) 0.025 0.01

Eastern Brown Snake (Pseudonaja textilis) 0.053 0.041

Northern Taipan (Oxyuranus scutellatus) 0.099 0.064

Eastern Mainland Tiger Snake (Notechis s. scutatus) 0.118 0.118

Reevesby Island Tiger Snake (Notechis ater niger) 0.131 0.099

Beaked Sea Snake (Enhydrina schistosa) 0.164 0.173

Western Mainland Tiger Snake (Notechis scutatus occidentalis) 0.194 0.124

Black Mamba (Dendroaspis polylepis) 0.32
http://www.qm.qld.gov.au/features/snakes/dangerous/index.asp
www.avru.unimelb.edu.au/avruweb/snakebi.htm
Monteecucco
http://uninews.unimelb.edu.au/articleid_1640.html
http://www.toxinology.com/fusebox.cfm?fuseaction=main.snakes.display&id=SN052
http://www.qm.qld.gov.au/features/snakes/seq/hydrophiidae.asp
http://www.qm.qld.gov.au/features/snakes/snakedetail.asp?TaxName=Pseudonaja+textilis
Not all snakes, even within the same family are dangerous in equal manner.
Aipysurus eydouxii (Marbled Sea Snake), living on fish eggs does not need strongly poisonous venom. Its toxicity is 40-100 smaller then other six species from the same family.

The story below shows that we know indeed much on this (or his family) snakes:
- its behaviour and feeding [1]
- the chemical composition of the venom (i.e. the mass spectrum) [3]
- the gene sequence of its venom [4]
- explanation, why this snake is not any more (in the course of evolution) so much dangerous [2]
These are four pieces of the story, by Dr Bryan Fry from University of Melbourne
1. http://uninews.unimelb.edu.au/articleid_1640.html
The simple life of snakes leads to easy antivenoms
[UniNews Vol. 13, No. 14 9 - 23 August 2004]
By Elaine Mulcahy
Deputy Director of the University of Melbourne's Australian Venom Research Unit, Dr Bryan Fry, said the study found big differences in the effectiveness of two different types of antivenom currently used for the treatment of sea snake bites.
The simple life of sea snakes has given them a unique bite which could have major medical implications, while the antivenom of the tiger snake may not be as useful as previously believed.
For example, the taipans and brown snakes are very closely related land snakes and have very similar venoms, but they have each specialised so much that they need different antivenoms. The taipan antivenom is not effective for treating brown snake bites and vice versa.
2. http://mbe.oxfordjournals.org/cgi/content/full/22/4/934
Putting the Brakes on Snake Venom Evolution: The Unique Molecular Evolutionary Patterns of Aipysurus eydouxii (Marbled Sea Snake) Phospholipase A2 Toxins
Min Li*, Bryan G. Fry , and R. Manjunatha Kini*,
Molecular Biology and Evolution 2005 22(4):934-941; doi:10.1093/molbev/msi077
* Department of Biological Sciences, Faculty of Science, National University of Singapore, Singapore; Australian Venom Research Unit, Department of Pharmacology, School of Medicine, University of Melbourne, Parkville, Victoria, Australia; Population and Evolutionary Genetics Unit, Museum Victoria, G.P.O. Box 666E, Melbourne, Australia; and Department of Biochemistry and Molecular Biophysics, Virginia Commonwealth University, Medical College of Virginia
Snake poisons block processes occurring on cellar membranes, including conducting nervous signals.
Unlike their mammalian counterparts, snake venom PLA2 enzymes are toxic and induce a wide variety of pharmacological effects including neurotoxic, cardiotoxic, myotoxic, anticoagulant, antiplatelet, convulsive, hemolytic, and hemorrhagic effects. Such an ability to induce diverse biological effects is due to their ability to interact with distinctly different target proteins located on the cell surface of the target tissues.
But this snake is not so much dangerous any more
The toxicity of A. eydouxii venom is 40-100 times lower than that of other sea snakes including the other six species in the same genus (Tu 1973, 1974). Similarly, the Australian terrestrial elapid Brachyurophis (shovel-nosed snakes) specializes in feeding upon reptile eggs (Scanlon and Shine 1988). The switch over to exclusive egg-feeding habit effectively removes the selection pressure to retain potentially toxic venom. Therefore, any change in toxin sequence (and hence bioactivity) would be a neutral mutation in these snakes as the toxins are not at all being used for prey capture.
We can even know, how this enzyme acts
In the present study, a very interesting finding is that the site of active site His48 in AY561155 is replaced with Arg48 (fig. 2). The proposed catalytic mechanism of PLA2 depends on the crucial role of His48; it polarizes and attracts a proton from a positionally conserved water molecule, which then participates in the formation of a tetrahedral intermediate. Upon collapse of the intermediate, hydrolysis products are released and three water molecules move into the active site (Scott et al. 1990). The substitution of His48 in AY561155 by Arg48 most likely disrupts the catalytic network, leading to significant, if not complete, reduction in the catalytic activity. This is the first natural substitution of active site His48 in PLA2 enzymes.
3. http://www3.interscience.wiley.com/cgi-bin/fulltext/104552321/HTMLSTART
Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: evolutionary and toxinological implications
Bryan G. Fry 1 2 *, Wolfgang Wüster 3, Sheik Fadil Ryan Ramjan 2, Timothy Jackson 1, Paolo Martelli 4, R. Manjunatha Kini 2
1 Australian Venom Research Unit, Department of Pharmacology, University of Melbourne, Parkville, Vic 3010, Australia
2 Department of Biological Sciences, Faculty of Science, National University of Singapore 119260, Singapore
3 School of Biological Sciences, University of Wales, Bangor LL57 2UW, Wales, UK4Veterinary Department, Singapore Zoo, Mandai Rd., Singapore
Rapid Communications in Mass Spectrometry
Volume 17, Issue 18, Pages 2047-2062
This mass spectrum shows how drastically venoms from single-species snakes differ, even within the same family.
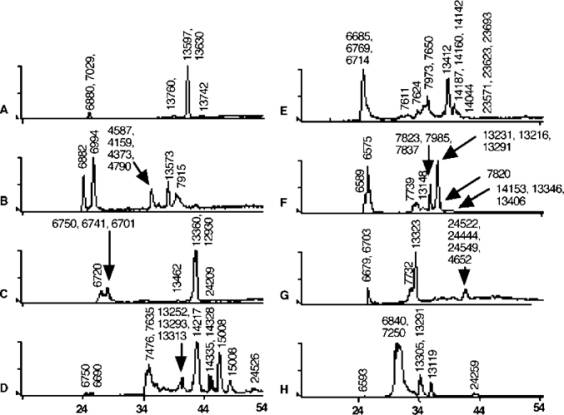
Figure 7. LC/MS analysis of marine Elapidae (A) Aipysurus duboisii, (B) Aipysurus foliosquama, (C) Aipysurus fuscus, (D) Lapemis curtus, (E) Enhydrina schistosa (Malaysia), (F) Enhydrina schistosa (Weipa, Australia), (G) Pelamis platurus, and (H) Laticauda schistorhynchus. X-axis is percentage of acetonitrile at time of elution; Y-axis is relative intensity (0-100%). Reconstructed mass in Daltons is shown above each peak.
Mass spectroscopy was performed with a novel, electrospray technique
On-line liquid chromatography/mass spectrometry (LC/MS) analysis of venom samples dissolved in 0.1% trifluoroacetic acid (TFA) to a concentration of 3 mg/mL was performed on a Phenomenex Jupiter C18 column (1 × 150 mm, 5 particle size, 300 A) with solvent A (0.05% TFA) and solvent B (90% acetonitrile in 0.045% TFA) at a flow rate of 50 L/min. The solvent delivery and gradient formation of a 1% gradient from 0-60% acetonitrile/0.05% TFA over 60 min were achieved using a Shimadzu LC 10AD solvent delivery system. Electrospray mass spectra were acquired using a PE-SCIEX API 300 system with a turbo ionspray atmospheric pressure ionization source. Samples (25 L) were injected manually into the LC/MS system and analyzed in positive ion mode. Full-scan data were acquired with the electrospray sprayer voltage at 4600 V, orifice 45 V and ring 350 V, over the m/z range 600-3000 with a step size of 0.2 Th. Data processing was performed with the aid of the software package Biomultiview (PE-SCIEX, Canada). Isolation, characterization and Edman degradation sequencing of venom components were performed as previously described.[10] Removal of pyroglutamic acid was accomplished using pyroglutamase (Takara Biomedicals, cat. no. 7334) following the standard protocol.
[4] Nucleotic sequence of genes responsible for venom production are deposited at NCBI
http://www.ncbi.nlm.nih.gov/
National Center for Biotechnology Information
http://www.ncbi.nlm.nih.gov/Database/index.html
Entrez is the integrated, text-based search and retrieval system used at NCBI for the major databases, including PubMed, Nucleotide and Protein Sequences, Protein Structures, Complete Genomes, Taxonomy, and others. Click on the graphic below for a more detailed view of Entrez integration.
http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=49472961
ORGANISM Aipysurus eydouxii
Eukaryota; Metazoa; Chordata; Craniata; Vertebrata; Euteleostomi;
Lepidosauria; Squamata; Scleroglossa; Serpentes; Colubroidea;
Hydrophiidae; Aipysurus.
DEFINITION Aipysurus eydouxii clone p115 phospholipase A2 isozyme mRNA, complete cds.
| 1 | gattcatctt | gcttgcagct | tcaccactga | caaaatgtat | cctgctcacc | ttctggtcct |
| 61 | cttggcagtt | tgtgtctccc | tcttaggagc | ctctgacatt | cctcctctgc | ctctgaacct |
| 121 | ctatcagttc | gacaacatga | ttcaatgtgc | caacaagcgc | aaaagagcta | cttggcatta |
| 181 | tatggactac | ggttgctact | gcggctgggg | aggtagcggg | acaccggtag | atgcgttgga |
| 241 | taggtgctgc | aaagtacatg | acgactgcta | tggtgtagcc | gaaaataacg | gatgctcccc |
| 301 | caagtggacg | ttgtatagtt | ggcaatgtac | tgaaaatgta | cccacctgcg | attcagaatc |
| 361 | ggggtgtgca | cttactgtgt | gtgcttgtga | cgccacagca | gccaagtgct | ttgccaaagc |
| 421 | cccttacaac | aacacgaact | acaatatcga | caccagtaat | tgccaatgat | atttgagagg |
| 481 | cttcagcgca | aggactgtgg | cagttactca | cctgcgcgtg | gcaattctct | ggacgggcct |
| 541 | ccattatata | tataataata | gaaaattata | tatatataat | tattaaaaaa | caaaaggaac |
| 601 | cgtttcctga | acaataaagt | gaggtgccga | caaaaaaaaa | aaaaaaaaaa | aa |