When
J.J. Thompson become chief of the Cavendish laboratory, in 1894, two talented
students arrived:
J.S. Townsend, a specialist in electrical discharges and E. Rutherford
from New Zealand.
In 1897 J.J. collects results on the cathode rays, and creates „own” model of the atom: a cake with raisins.
The
student Rutherford was not convinced by this model, but he did not argue with a proffesor.
He made his own experiment far away, in Montreal, in 1911.
E. Rutherford studied the anglular distribution of alpha particles passing through a golden foil (gold can be hammered into the very thin plates). The measured angular distribution corresponded to the scattering on a point charge. It was a lucky coincidence: Coulomb’s potential is the only one which in classical and quantum models gives the same angular distribution!

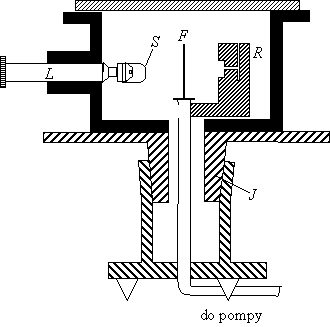
Experimental set-up for scattering of aparticles: they are emitted from a radioactive source R and are diffracted by gold foil F. The chamber is kept under vacuum and the observation lens can be rotated around the foil axis, J is a rotable vacuum seal. L is a spintariscope, invented by Crookes in 1903.
Alpha particles from polon were used in other, important
discoveries:
- artificial radioactivity ,
- neutron.