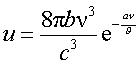
Max Planck, a theoretical physics professor in Berlin, decided at the dawn of
XIX century to explain how Kirchoff’s laws of emmision and absorbtion of electromagnetic
radiation come from the second principle of thermodynamics.
Travelling to India he discovered America.
In 1899 Planck [1] obtained an energy-density u distribution vs. wavelength (i.e. frequency v) in a quite good agreement with the existing experimental data.
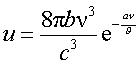
Just when Planck announced with triumph his results, malicious voices in Berlin started to whisper, that new experimental data, in far infrared (at 50 micron), are not in accord with his “law”. At first October Sunday of 1900 the experimentalis H. Rubens (and others cited [2]) send to Planck their final results. Planck spent hectic two month trying to fit with an analytical formula those data.
14 December 1900 on German Physics Society meetinghe showed a new formula, for which the previous one was only an approximation
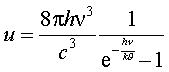
To obtain this formula Planck had to assume that the energy appears in “parts” proportional to the light frequency v: E=hv with constanth=6.55•10-34 Js.
Planck did not use the term “quant”, althrough he knew this word, what is clear from his article [3], just next page after the work [2]. Even in 1913 on the “Solvay” Congress he continued to insist that "photon" is a wrong hipothesis.
[1] Ueber irreversible Strahlungsvorgänge, Annalen der Physik IV, 1 (1900)
69
[2] Ueber das Gesetz der Energieverteilung im Normalspektrum, Ann. der
Physik IV, 4 (1900) 553
[3] Ueber die Elementarquanta der Materia und Elektricitat, Ann. der Physik
IV, 4 (1901) 564