The electron first got its name and only later has been officially discovered. With its Greek meaning amber, the name "electron" was used by G. Johnstone Stoney in 1891 to define "the atom of electricity", carried in electrolysis. But JJ Thompson, who made decisive experiments, in his Nobel speach in 1906 still did not use the name "electron".
JJ Thompson wanted to know if mysterious cathode rays, known for more than twenty years, were ethereal vibrations (like Roentgen rays, or Hertz's signals) or material particles.
In three experiments JJ Thompson showed that corpuscles:
1) the cathode rays were bent by magnetic fields, like it happens in (traditional) TV sets

2) they were bent by electric fields, like in (old) oscilloscopes
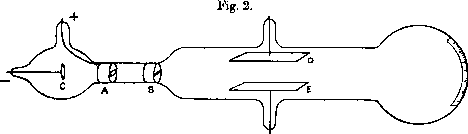
3) they were bringing a negative electrical charge, making the TV screen electrised (and attract dust).
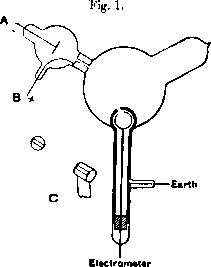
JJ Thompson also explained, how these primordial atoms may be organized into electrically neutral chemical atoms.
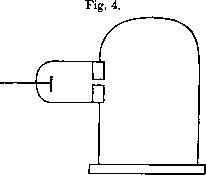
Still in 1906, in his Nobel prize lecture, JJ Thompson used the name "corpuscles" and not "electrons".
J. J. Thomson, Cathode Rays, Philosophical Magazine, 44 (1897) 293
http://web.lemoyne.edu/~giunta/thomson1897.html
J. J. Thomson, Carriers of Negative Electricity, Nobel Lecture, December 11, 1906
http://nobelprize.org/nobel_prizes/physics/laureates/1906/thomson-lecture.pdf