Electron attachment in
DNA
Cosmic radiation, both primary (protons with energy range from 10-7 to 10-20 eV, positrons, electrons, gamma radiation) and secondary (mainly mions and electrons created in their decay) causes biological damages of different kinds, among others in the DNA structure (mutations, genotype recombinations, denaturation of DNA). However it seems, that these damages are not caused by the radiation itself, but mainly by electrons created in living cells in ionization events, for example particles of water. Energy of such electrons is in range from 1 to 20 eV, what theoretically is not sufficient to ionize complex molecules (for example the ionization threshold for ethyl alcohol is equal to 10.5 eV). Until recent time it was not sure, if such low energy electrons can cause genetic damages of DNA, like SSB (Single Strand Break) - breake of one DNA helix or DSB (Double Strand Break) - breaking both strips of the helix.
Canadian scientists [Science, Vol 287, 3/12/2000] performed a complicated experiment, which have to explain mechanisms of these damages. The results were surprising (Fig. 1):
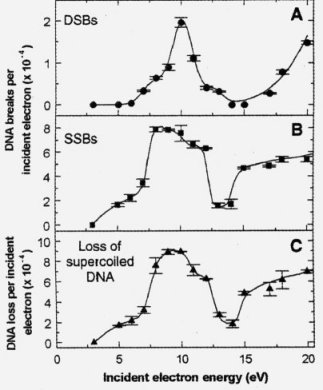
DNA structure damages caused by low-energy electrons.
1. Very low energy electrons cause DNA damages (both SSB and DSB) even at energies below ionization threshold of DNA (from 7.5 up to 10 eV).
2. Capacity of the damage to DNA (this capacity is called "cross section") strongly depends on electrons energy. It means that it has a resonance character. The observed reaction threshold is about 3 eV for SSB and about 5 eV for DSB; a maximum of the cross section is about 10 eV. This behaviour differs from the interaction of electromagnetic quanta (photons) with DNA. In this case we can see a monotonic increase of the number of the damages from 7 to 12 eV, and next, for bigger energies a constant cross section up to 2 keV.
3. The observed maximum of the cross sections is about twice in magnitude that for photons.
These result show that the damage depends not only on the
energy of the absorbed quanta, but also on the nature of its carrier.
The explanation of this process assumes an electron attachment
to the molecule and a creation of the resonance state - a temporary molecular
anion
e-+ RH = RH*-,
which subsequently decays by auto-detaching of the electron
or by dissociation (with detaching of the H- ion), cracking
along one or more bonds
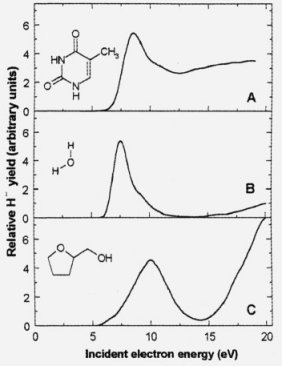
Electron-induced damages in molecules in a condensed film phase (A - thymine, B - water, C - tetrahydrofuryl alcohol).